
Have you ever wondered how your drinking water remains clear and safe, or how your garden blooms with vibrant colors? The answer might surprise you: aluminum sulfate. Often overlooked, this compound plays a pivotal role in various industries, including horticulture, water treatment, and manufacturing. In this blog, we'll delve into the fascinating world of aluminum sulfate, exploring its chemistry, myriad applications, and the best practices for its use.
Aluminum sulfate, commonly referred to as alum, is a chemical compound with the formula Al 2 (SO 4 ) 3 . It appears as white, lustrous crystals or powder and is renowned for its ability to purify water by causing impurities to clump together, making them easier to filter out. This characteristic is crucial in municipal water treatment facilities, ensuring the water flowing from your tap is clean and safe to drink ( Affinity Chemical ).
In horticulture, aluminum sulfate is a gardener's ally, used to adjust soil pH levels. By making soil more acidic, it helps certain plants, like hydrangeas, achieve their desired color. Imagine your garden bursting with blue hydrangeas, a direct result of this compound's application! Moreover, its use extends to the textile industry, where it acts as a dye fixer, preventing colors from washing out of fabrics ( Aluminum Manufacturers ).
This blog aims to provide you with essential insights into the chemistry and applications of aluminum sulfate. We'll discuss safety measures and best practices to ensure its effective use. Whether you're a professional in an industrial setting or a home gardener, understanding aluminum sulfate can enhance your processes and outcomes. Stay tuned as we explore the depths of this versatile compound.
What is aluminum sulfate, and why is it so widely used across various industries? At its core, aluminum sulfate is an inorganic compound with the chemical formula Al 2 (SO 4 ) 3 . This compound is often found as a white, crystalline solid, available in either granule or powder form. Its solubility in water makes it particularly useful for applications that require a quick reaction ( Wikipedia ).
Aluminum sulfate's significance stems from its versatile properties. It is a highly effective coagulant, which means it helps particles in liquids clump together, making them easier to remove. This property is particularly beneficial in water treatment processes, where it aids in purifying drinking water by precipitating impurities. Imagine a cloudy glass of water becoming clear as particles settle at the bottom—a transformation facilitated by aluminum sulfate ( Affinity Chemical ).
In terms of physical appearance, aluminum sulfate can be described as a white, lustrous crystalline solid. It is known for its ability to dissolve readily in water, forming a solution that can be used in various chemical processes. The industrial-grade forms of aluminum sulfate often include varying levels of hydration, which can affect its solubility and application methods. For example, the anhydrous form is less common, while the hexadecahydrate form is more prevalent in industry due to its ease of handling and solubility ( Affinity Chemical ).
Beyond its role in water treatment, aluminum sulfate is also a key player in the textile industry. It serves as a mordant in dyeing processes, helping dyes adhere more effectively to fabrics. This property makes it invaluable in ensuring that colors remain vibrant and do not wash out easily. Furthermore, in the paper manufacturing sector, aluminum sulfate is used to control the pH of the paper pulp, improving the quality and durability of the final product.
Overall, the properties of aluminum sulfate—its solubility, coagulant nature, and ability to interact with other substances—make it a versatile and essential compound in various industrial applications. As we continue exploring its uses, you'll find that understanding these fundamental properties is crucial to leveraging aluminum sulfate effectively in both professional and personal settings.
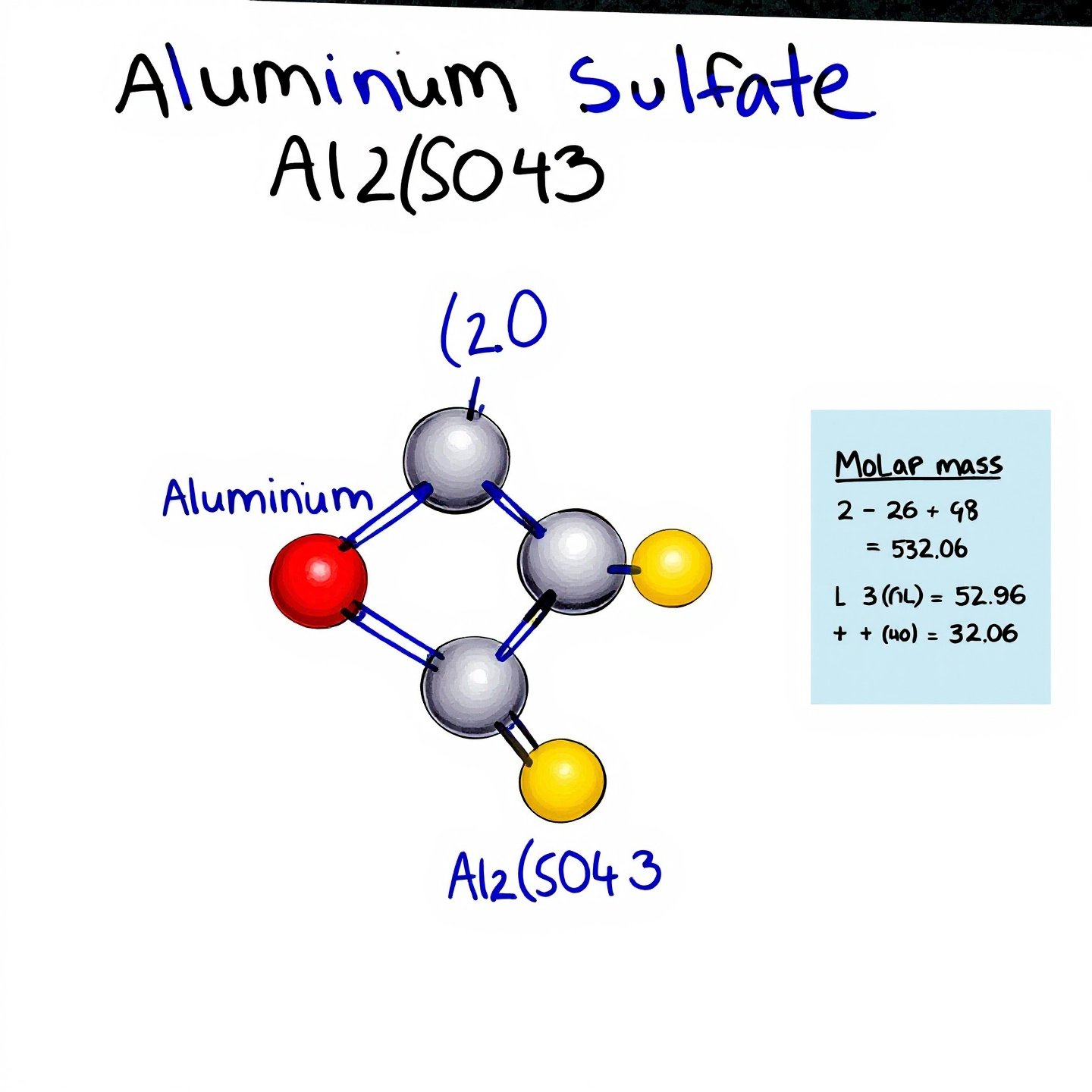
When you hear the term "aluminum sulfate," it might sound complex, but breaking down its chemical formula can provide a clearer understanding of its structure and applications. The formula for aluminum sulfate is Al 2 (SO 4 ) 3 . This notation indicates that each molecule consists of two aluminum (Al) atoms, three sulfur (S) atoms, and twelve oxygen (O) atoms. This composition is crucial for understanding how aluminum sulfate interacts in various chemical processes.
Calculating the molar mass of aluminum sulfate is a practical exercise that helps in determining the amount needed for specific applications, such as in water treatment or soil amendment. The molar mass is essentially the mass of one mole of a substance, expressed in grams per mole (g/mol). For aluminum sulfate, the molar mass is calculated as follows:
Adding these values gives the total molar mass of aluminum sulfate: 342.1509 g/mol ( WebQC ).
Understanding the molar mass is vital for practical applications. For instance, in water treatment facilities, precision is key. Knowing the exact molar mass allows for accurate dosing, ensuring that the coagulation process is effective without overuse, which could lead to unnecessary costs or potential environmental impacts. Similarly, in horticulture, the correct amount of aluminum sulfate can adjust soil pH effectively, promoting healthy plant growth without damaging the soil ecosystem.
Imagine you're tasked with preparing a solution for a large-scale water treatment operation. By understanding the molar mass, you can calculate the exact amount of aluminum sulfate needed to achieve the desired concentration, ensuring optimal performance and cost-efficiency.
In conclusion, the chemical formula and molar mass of aluminum sulfate are not just theoretical concepts but practical tools that facilitate its effective use in various industries. As we move forward, keep these calculations in mind, as they are fundamental to harnessing the full potential of this versatile compound.
When it comes to aluminum sulfate, there are several key variants that serve distinct purposes in various industries. Among these, sodium aluminum sulfate and potassium aluminum sulfate stand out for their unique applications and benefits.
Sodium aluminum sulfate (SAS), also known as sodium alum, is primarily used in the baking industry. It acts as a slow-acting leavening acid, which is essential in the production of baked goods like cakes and muffins. This compound reacts with baking soda under heat to release carbon dioxide, helping dough rise evenly during baking ( Food Additives ).
Despite its effectiveness, SAS is not widely used on its own due to its slow reaction rate at room temperature. Instead, it is often combined with faster-acting acids like monocalcium phosphate to form double-acting baking powders. This combination ensures that the leavening process occurs both at room temperature and during baking, providing a consistent texture in baked goods.
Potassium aluminum sulfate, commonly known as potassium alum, is another variant with diverse applications. One of its most intriguing uses is in crystal deodorants. Potassium alum is valued for its natural antimicrobial properties, which help reduce odor-causing bacteria without blocking sweat glands. This makes it a popular choice for those seeking natural deodorant alternatives ( Healthline ).
Beyond personal care, potassium aluminum sulfate is also used in water purification and as a mordant in dyeing processes. Its ability to help dyes adhere to fabrics ensures vibrant, long-lasting colors, making it indispensable in the textile industry.
In conclusion, the variations of aluminum sulfate, such as sodium and potassium forms, highlight the compound's versatility. Each variant brings unique benefits to its respective field, from enhancing the texture of baked goods to providing natural personal care solutions. As we continue to explore aluminum sulfate, understanding these variants and their applications can provide deeper insights into its widespread use.

When it comes to nurturing a thriving garden or agricultural landscape, understanding soil chemistry is crucial. Aluminum sulfate plays a significant role in adjusting soil pH, making it an invaluable tool for gardeners and farmers alike. But how exactly does this compound work, and what are the best practices for its use?
Soil pH is a critical factor that influences nutrient availability and plant health. Many plants, such as azaleas and blueberries, thrive in acidic soils. Aluminum sulfate is frequently used to lower the pH of alkaline soils, creating a more acidic environment conducive to the growth of these acid-loving plants. When aluminum sulfate is applied to soil, it reacts to form sulfuric acid, which effectively lowers the soil pH ( Purdue Extension ).
To determine the appropriate amount of aluminum sulfate needed, a soil test is essential. This test provides a baseline pH level, guiding the application process. For instance, if the soil pH is above 7.0, an application of aluminum sulfate can help bring it down to the desired 5.5 range, which is ideal for many acid-loving plants ( Hi-Yield Aluminum Sulfate ).
Correct dosage and application are vital to avoid potential adverse effects. For small garden plots, the general recommendation is to use approximately 5 pounds of aluminum sulfate per 100 square feet of soil. This should be thoroughly mixed into the soil and watered in to ensure even distribution. For larger areas or different soil types, adjustments may be needed. For instance, sandy soils require less aluminum sulfate, while clay soils may need more to achieve the same pH change ( Purdue Extension ).
Aluminum sulfate can be applied either as a dry powder or dissolved in water. When using it in liquid form, a common practice is to mix 1 pound of aluminum sulfate in 5 gallons of water, then apply it evenly across the soil surface. This method ensures quick absorption and minimizes the risk of harming plant roots.
While aluminum sulfate is effective in modifying soil pH, it must be used with caution. Over-application can lead to excessively acidic soils, which may harm plants not adapted to such conditions. Additionally, excessive aluminum in the soil can become toxic to plants, affecting root growth and nutrient uptake. Therefore, it is crucial to follow recommended guidelines and re-test soil pH periodically to ensure optimal growing conditions.
In conclusion, aluminum sulfate is a powerful tool for managing soil pH and enhancing plant health. By understanding its properties and applying it correctly, gardeners and farmers can create optimal growing environments, leading to healthier plants and more productive gardens.
Imagine walking through a garden bursting with vibrant blue hydrangeas and lush, healthy plants. This picturesque scene can be achieved with the help of aluminum sulfate, a versatile compound that not only enhances the color of hydrangeas but also boosts overall garden productivity. But how exactly does this work?
Hydrangeas are unique in that their bloom color can be influenced by the pH of the soil. Aluminum sulfate plays a crucial role in this transformation. By lowering the soil's pH, it facilitates the absorption of aluminum ions by the hydrangea plant, which, in turn, results in the iconic blue hue of the blooms. If your hydrangeas are pink and you desire that striking blue, adjusting the soil pH with aluminum sulfate is your solution ( Mississippi State University Extension ).
To achieve the desired bloom color, the following steps can guide you:
Beyond hydrangeas, aluminum sulfate can enhance garden productivity by improving soil conditions for a variety of acid-loving plants. By lowering soil pH, it increases nutrient availability, supporting robust plant growth. This is particularly beneficial for plants like azaleas and blueberries, which thrive in acidic environments.
However, it's crucial to avoid over-application, which can lead to overly acidic soils and potential plant damage. Always start with small amounts and increase gradually, keeping a close eye on plant health and soil conditions.
In summary, aluminum sulfate is a powerful ally in transforming your garden into a vibrant, thriving ecosystem. By understanding and applying it correctly, you can enjoy the beauty of blue hydrangeas and a productive garden filled with healthy, flourishing plants.

Imagine diving into a pool with water so clear, it feels like you're swimming in liquid glass. Achieving this level of clarity often involves the use of aluminum sulfate, a powerful tool in the arsenal of pool maintenance. But how does aluminum sulfate work its magic in pools, and what are the best practices for its use?
Aluminum sulfate, commonly known as alum, is prized for its ability to clarify pool water by acting as a coagulant and flocculant. When added to pool water, it triggers a chemical reaction that forms a gelatinous precipitate. This substance effectively traps fine particles like dirt and algae, which are then easily captured by the pool's filtration system, resulting in sparkling clear water ( Shijiazhuang Yuncang Water Technology ).
The process of using aluminum sulfate for pools, often referred to as "floccing," involves several steps. Initially, the alum is broadcast over the pool surface, where it interacts with suspended particles, causing them to clump together. These clumps, being heavier than water, settle at the bottom of the pool, where they can be vacuumed away. This method is particularly effective for clearing cloudy or murky water, turning it pristine without the need to drain the pool ( In The Swim ).
For optimal results, it's crucial to use the correct dosage of aluminum sulfate. The general guideline is to use about 4 pounds of alum per 10,000 gallons of pool water. After application, the pool's pump should be run for a couple of hours to circulate the alum, then turned off to allow the particles to settle. The next day, the settled material should be vacuumed to waste. It's important to ensure that your pool's filtration system is capable of handling this process, as some filters may become clogged if not properly managed.
Handling aluminum sulfate requires care. Always wear protective gloves and eyewear to avoid irritation. Additionally, maintaining a balanced pool pH is essential, as alum works best in water with a pH level between 7.0 and 7.8. Regular monitoring and adjustments will help maintain the health of your pool and its equipment.
Incorporating aluminum sulfate into your pool maintenance routine can transform your swimming experience, ensuring clear, inviting water. As you enjoy your pristine pool, remember that understanding and applying the right techniques is key to achieving the best results. Next, we'll explore the fascinating chemical reactions of aluminum sulfate and its bromide compounds, revealing even more about this versatile compound.
When diving into the world of chemistry, it's fascinating to explore the reactions and compounds that emerge from aluminum sulfate interactions. One such compound is aluminum sulfate bromide, which plays a unique role in chemical processes. But what exactly happens when aluminum sulfate reacts with other substances, and how can we understand these reactions?
Aluminum sulfate is known for its ability to participate in various chemical reactions, particularly double displacement (metathesis) reactions. A classic example involves its reaction with ammonium bromide (NH 4 Br) to form aluminum bromide (AlBr 3 ) and ammonium sulfate ((NH 4 ) 2 SO 4 ). The balanced chemical equation for this reaction is:
6NH 4 Br + Al 2 (SO 4 ) 3 → 2AlBr 3 + 3(NH 4 ) 2 SO 4
This reaction exemplifies how aluminum sulfate can transform into different compounds under the right conditions, showcasing its versatility in chemical synthesis ( Chemical Aid ).
Aluminum sulfate bromide, while not as commonly discussed, is an intriguing compound formed through the interaction of aluminum sulfate with bromide ions. This compound can be utilized in various industrial applications, particularly where specific bromide characteristics are desired.
To better understand these reactions, consider the thermodynamic properties involved. For instance, the reaction between NH 4 Br and Al 2 (SO 4 ) 3 is endothermic, meaning it absorbs heat, and is endoentropic, indicating an increase in entropy. These properties highlight the energetic shifts and molecular rearrangements that occur during the reaction, providing insight into the conditions necessary for successful synthesis.
| Element | Reactants | Products | Balanced? |
|---|---|---|---|
| Nitrogen (N) | 6 | 6 | ✔️ |
| Hydrogen (H) | 24 | 24 | ✔️ |
| Bromine (Br) | 6 | 6 | ✔️ |
| Aluminum (Al) | 2 | 2 | ✔️ |
| Sulfur (S) | 3 | 3 | ✔️ |
| Oxygen (O) | 12 | 12 | ✔️ |
This table illustrates how each element is balanced in the reaction, ensuring that the law of conservation of mass is upheld. Understanding these balances is crucial for chemists and industry professionals who rely on precise chemical reactions for product development and manufacturing.
In summary, the exploration of aluminum sulfate reactions, particularly those involving bromide compounds, reveals the complexity and potential of this versatile chemical. These reactions not only enhance our understanding of aluminum sulfate's capabilities but also open doors to innovative applications in various fields. As we continue our journey, we'll next delve into the best practices and safety measures necessary for handling aluminum sulfate effectively.
Handling aluminum sulfate requires a careful approach to ensure safety and minimize environmental impact. As a designated hazardous substance under the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA), it is crucial to follow established guidelines for its use ( Affinity Chemical ).
When handling aluminum sulfate, personal protective equipment (PPE) is essential. This includes wearing gloves, goggles, and protective clothing to prevent skin and eye contact, which can cause irritation or burns. In case of skin exposure, immediately remove contaminated clothing and rinse the affected area with water for at least 15 minutes. For eye exposure, flush with water and seek medical attention if irritation persists ( Affinity Chemical ).
Storage of aluminum sulfate should be in a cool, dry place, away from heat sources and direct sunlight. Stainless steel or fiberglass containers are recommended to prevent reactions with other materials. Ensure that storage areas are well-ventilated and free from incompatible substances, such as hypochlorites, which can produce hazardous byproducts.
Proper disposal of aluminum sulfate is critical to prevent environmental contamination. Spills should be cleaned promptly, with dry spills swept into a covered container and residues washed down with water. For liquid spills, absorb with sand or vermiculite and dispose of in accordance with local regulations. Avoid flushing aluminum sulfate into sewer systems or waterways to prevent ecological damage ( ASEDA Chemicals ).
Aluminum sulfate can affect aquatic ecosystems by altering water pH and harming aquatic life. Therefore, industries must treat wastewater before discharge, ensuring compliance with environmental standards to minimize impact.
In the event of an accidental release, isolate the area and use appropriate fire-fighting measures if necessary. Although aluminum sulfate is not combustible, it can decompose at high temperatures, releasing sulfur trioxide, which supports combustion. Use suitable extinguishing agents for surrounding fires and avoid inhaling combustion byproducts.
By adhering to these safety measures and handling guidelines, the risks associated with aluminum sulfate can be effectively managed. This ensures not only the safety of personnel but also the protection of the environment, allowing for the responsible use of this versatile compound.
As we wrap up our exploration of aluminum sulfate, it's clear that this compound is a cornerstone in many industries, from water treatment to horticulture. Understanding its chemical properties and applications allows us to harness its full potential safely and effectively. Whether you're adjusting soil pH for vibrant hydrangeas or ensuring crystal-clear pool water, aluminum sulfate proves to be a versatile ally.
Throughout this blog, we've delved into its various uses, from acting as a coagulant in water purification to its role in enhancing the color of blooms in your garden. The key to maximizing these benefits lies in informed usage. Always adhere to recommended dosages and safety guidelines to mitigate any potential risks. Proper handling and storage, as well as understanding the environmental implications, are crucial steps in responsible usage.
Additionally, as you consider the broader applications of aluminum sulfate, it's worth noting the expertise of industry leaders like Shengxin Aluminum. As a premier manufacturer of aluminum profiles, Shengxin offers high-quality solutions that complement the use of aluminum sulfate in various sectors. Their commitment to innovation and excellence ensures that you'll receive top-notch products and services ( Shengxin Aluminum ).
In conclusion, aluminum sulfate is more than just a compound; it's a key player in enhancing processes and outcomes across multiple domains. By applying the insights gained here, you can make informed decisions that benefit both your projects and the environment. We invite you to continue exploring the possibilities with Shengxin Aluminum, where expertise meets innovation.
Aluminum sulfate can cause local tissue irritation upon contact with skin or eyes and may form sulfuric acid when hydrolyzed. It's not classified as a carcinogen, but proper handling and protective gear are recommended to avoid irritation.
Yes, aluminum sulfate is generally recognized as safe (GRAS) by the FDA when used in food as a firming agent, sequestrant, or pH adjuster. It's commonly used in pickling and baking practices.
Aluminum sulfate lowers soil pH, enabling hydrangeas to absorb aluminum ions, which turns their blooms blue. Proper application ensures vibrant colors without harming the plants.
In pools, aluminum sulfate acts as a flocculant, helping to clarify water by causing particles to clump together and settle, making it easier to filter out impurities.
Use personal protective equipment like gloves and goggles to prevent irritation. Store it in a cool, dry place and follow proper disposal methods to minimize environmental impact.
 Інтернет-сервіс
Інтернет-сервіс 0086 136 3563 2360
0086 136 3563 2360 sales@sxalu.com
sales@sxalu.com +86 136 3563 2360
+86 136 3563 2360